
Topical and Transdermal Product Development
Formulation Development
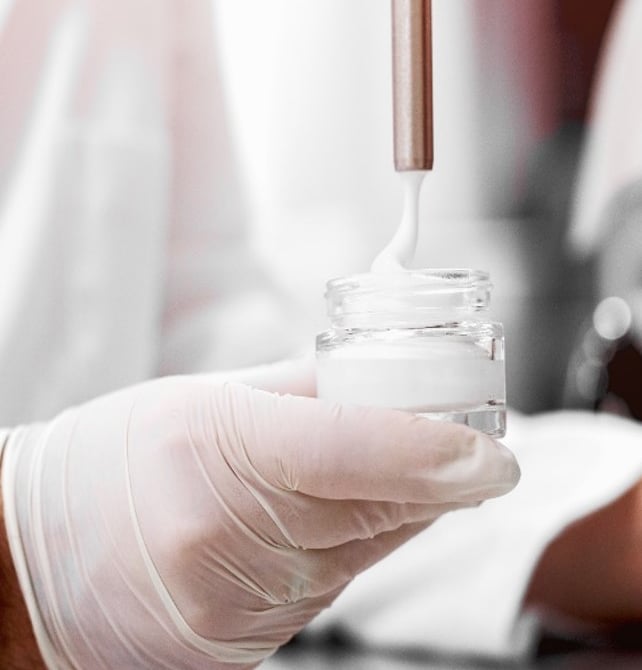
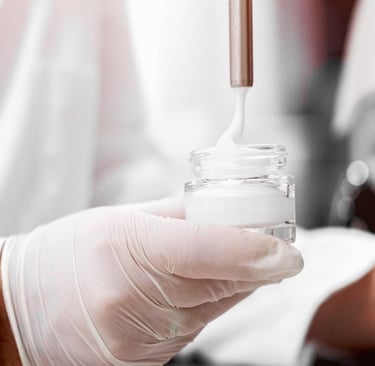
Topical and transdermal formulation development is a critical area of pharmaceutical research and development, focusing on the design and development of medications applied to the skin. These formulations offer an alternative to oral or injectable routes, providing localized or systemic effects through the skin.
Transkin performs multiple steps to develop effective and potential formulations.
We select excipients from the IID (Inactive Ingredient Database) that can reduce the risk of formulation irritation and improve patient compliance.
We can perform pre-formulation studies, such as solubility and stability studies
We can develop all kinds of formulations, such as Gel, Cream, Ointment, Patch, Lotion, Spray, Nail Lacquer, etc.
We can screen the permeation enhancers to improve the topical, transdermal, nail, and transcorneal delivery of drugs
We can perform formulation characterizations, including appearance, color, odor, rheological behavior, oil globule size, pH, specific gravity, etc.
Formulation Testing
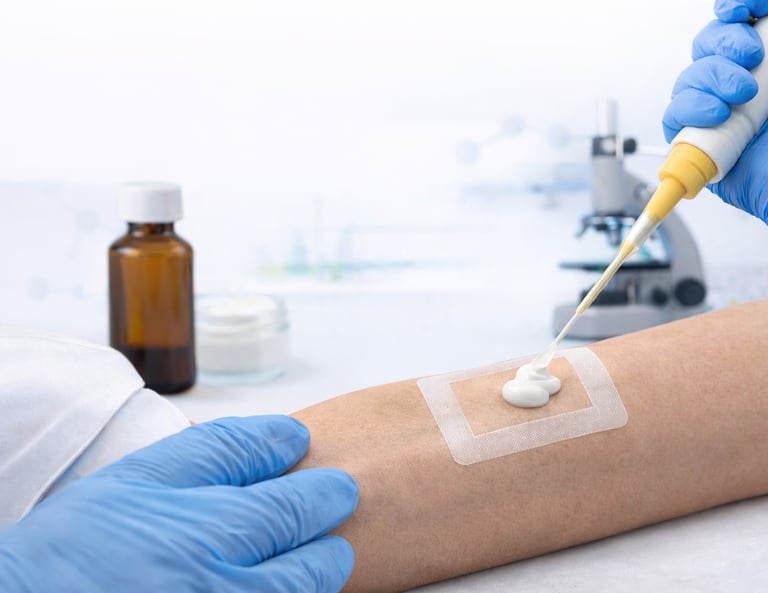
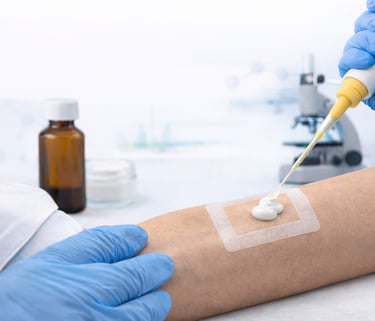
In Vitro Release Testing (IVRT)
In Vitro Release Testing (IVRT) is a laboratory procedure used to evaluate the rate and extent of drug release from topical and transdermal formulations, such as creams, gels, ointments, and patches. This testing simulates how a drug is released from its formulation when applied to the skin, providing critical information about its performance before in vivo testing or clinical trials.
IVRT is well-established for characterizing and evaluating the performance of semi-solid dosage forms. IVRT is a valuable tool for demonstrating comparative in vitro drug release rates between the test and reference products (FDA). We perform IVRT studies across various synthetic membranes, including polysulfone, cellulose acetate/nitrate mixed ester, Polytetrafluoroethylene, and polycarbonate.
In Vitro Permeation Test (IVPT)
An In Vitro Permeation Test (IVPT) study is a laboratory method used to evaluate the permeability and rate of drug absorption through the skin from topical formulations. These studies are conducted using human or animal skin samples mounted on diffusion cells to simulate the human skin barrier.
IVPT is performed to predict the drug's cutaneous pharmacokinetics. We perform IVPT studies across various biological membranes, including human cadaver skin, porcine skin, nail, and cornea, under various conditions (finite vs. infinite dosing, occluded vs. nonoccluded) using Franz diffusion cells.


Analytical Services


Accelerated and long-term physical and chemical stability studies of the formulations.
Force degradation studies and identification of degradants.
Drug degradation studies in contact with skin.
Analytical method development, validation, and testing.
Method validation is performed according to the ICH guidelines
Quantification of APIs in the epidermis, dermis, and receptor fluid.
Estimation of APIs in the plasma samples.
Address
12, Sudama Suncity, Ayodhya Bypass Rd, Piplani, Bhopal, Madhya Pradesh 462022, India
Contacts
+91 99819 83734
info@transkinresearch.com


